Gene therapy medications represent a groundbreaking advancement in the medical field, offering a novel and cutting-edge approach to treating various diseases and conditions at their genetic roots. These medications hold the promise of delivering long-lasting and even permanent cures for conditions that were previously deemed incurable, including various forms of cancer and hereditary disorders. However, the therapies do not come at a small price tag and may not always be effective. As employer plan sponsors, it is crucial to comprehend the magnitude of these treatments and their potential advantages and consequences for plan participants.
The History of Gene Therapy
The gene therapy industry has been gathering steam for decades. The global gene therapy market accounted for $2.05 billion in 2020 and is projected to be $12.29 billion by 2030.1 The history of gene therapy dates back to the 1960s, when scientists first hypothesized that DNA sequences could help patients treat genetic disorders.2 Today, there are six approved gene therapies with dozens more projected to launch throughout the decade including potential therapies for Duchenne muscular dystrophy and hemophilia A.2,3
Historically, most gene therapies are used to treat the rarest of conditions where the effects can last 5-to-20 years. Moving forward, gene therapy will be used to target both common and rare diseases offering “new hope for a different type of treatment”.4 Products in the pipeline will expand treatment options for familiar diseases such as Parkinson’s and osteoarthritis for the broader population. With these latest advancements, patients with chronic conditions will have gene therapy alternatives that offer efficacy and consistency.
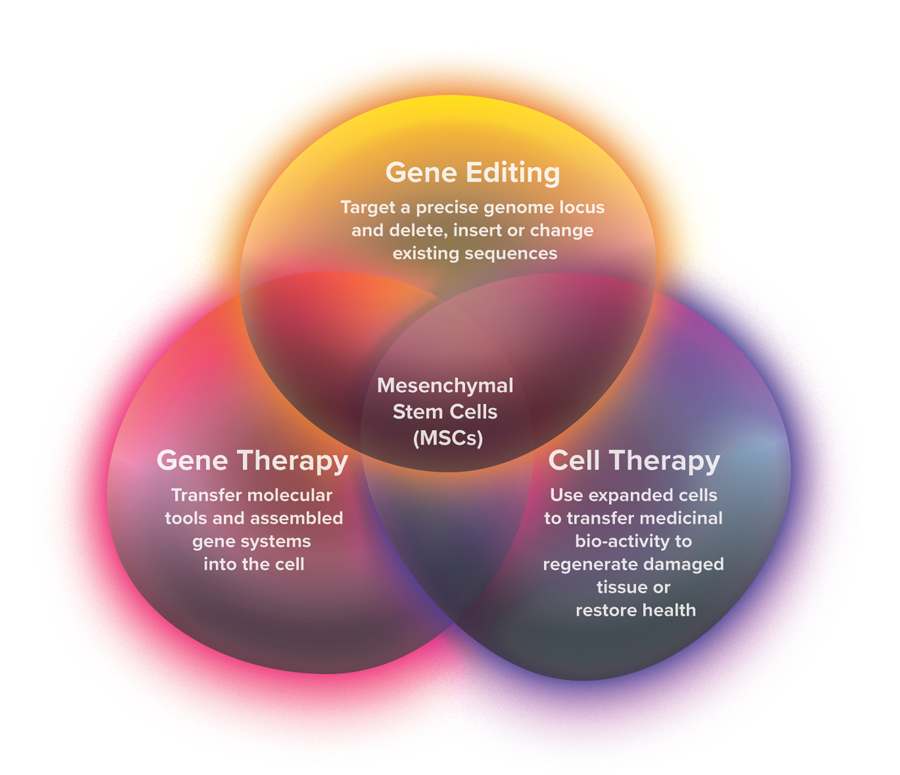
As seen in FIGURE 1, gene therapy, gene editing and cell therapy are fields of research with similar goals of treating various disease states by altering the blueprints at the microscopic level. Gene therapy transfers genetic material via a vector, or a carrier molecule, that delivers new healthy genetic content to a location where it can be utilized to counteract the effects of the mutated gene. Gene editing involves modifying and manipulating the genomic sequence to directly target and add, correct or remove harmful genes. Cell therapy involves administering viable cells into a patient’s body to replace the malfunctioning and damaged cells but does not have any effect on the genes.5
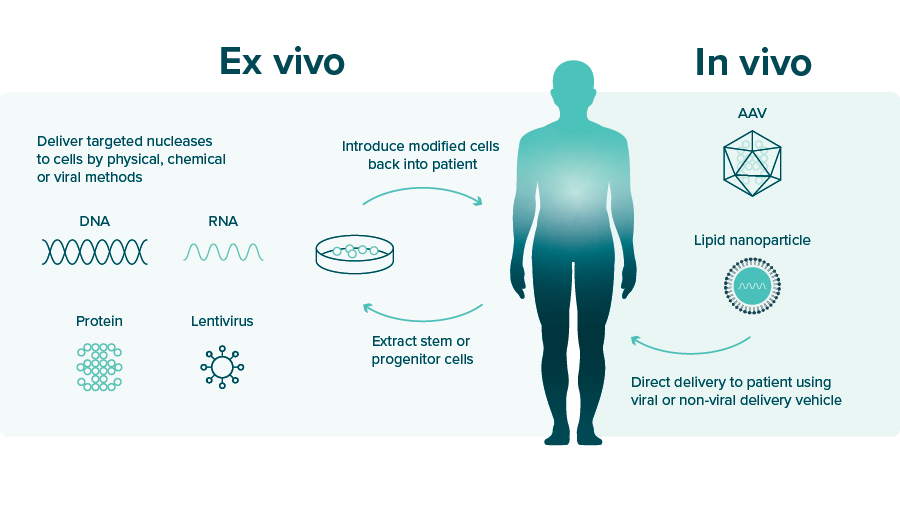
There are two main approaches to gene therapy: ex vivo and in vivo, pictured in FIGURE 2. In ex vivo, the target cells are removed, genetically altered and placed back into the patient. This approach is safer but is limited in that it can only be used when the disease allows the target cells to be removed. With in vivo, genetic material is administered directly to the target cells in the body through blood circulation or cerebrospinal fluid.6 The procedure is simple, but it is more challenging to reach the targeted cell. These therapies are profoundly effective in treating patients, but they are also extraordinarily expensive.6
Gene Therapy Cost and Availability
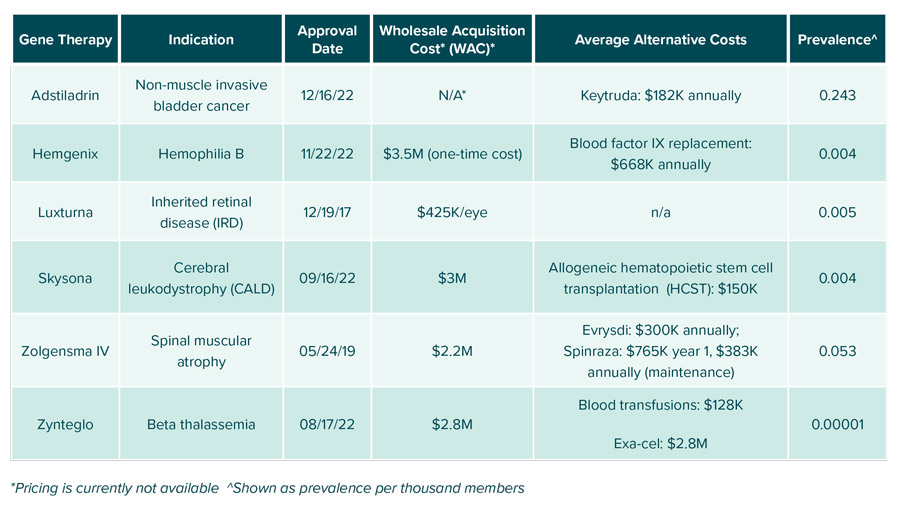
Currently, six FDA-approved gene therapies are available. details these six therapies as well as the cost of how these conditions are primarily treated.
The most recently approved gene therapy, Adstiladrin, should be commercially available in the second half of 2023. It is the first gene therapy indicated for non-muscle invasive bladder cancer that will be directly competing with Merck’s Keytruda. The pricing will not be published until closer to launch, but the manufacturer is aiming to make the therapy widely accessible. It is unique in that it will be administered once every three months for a total of four administrations. For invasive cancers, the response rate of 51% is strong, but it does give pause on what happens when the product is not effective.
Just a month before Adstiladrin’s approval, Hemgenix was approved by the FDA. It is indicated for hemophilia B, an extremely rare hereditary bleeding disorder caused by low levels of blood clotting Factor IX. Patients with hemophilia B experience spontaneous or excessive bleeding that can be life-threatening. Before approval of Hemgenix, the standard of care for hemophilia B consisted of episodic infusion therapy for mild or moderate and regular infusion for individuals with severe symptoms. As seen in FIGURE 3, Hemgenix is a one-time single-dose intravenous (IV) infusion that costs $3.5 million, however the cost benefits of this medication are seen long term as prophylactic blood infusions average about $668K annually per patient. It had an efficacy rate of 94% in clinical trials, so the vast majority of patients will see a benefit.
Unlike Hemgenix, Luxturna lacks alternative treatment options. It is the first FDA-approved gene therapy to treat patients with inherited retinal disease (IRD), which ultimately leads to progressive vision loss and potentially results in total blindness. Luxturna has demonstrated relatively safe and modest efficacy that varies by patient in terms of how substantial an improvement in vision is achieved.
Skysona received FDA approval last year for early active cerebral adrenoleukodystrophy (CALD), for boys 4-to-17 years old to slow the progression of neurologic decline. Progressive neurologic symptoms of CALD include hearing loss, diminished vision, gait instability, stiffness and seizures. If left untreated, within 2-to-3 years, symptoms can cause most neurologic function to be lost and total disability or even death. Current treatment available that serves as an alternative to Skysona is allogeneic hematopoietic stem cell transplantation (HSCT), but it requires a matching sibling donor and only about 30% of patients are a match.8 Thus, the estimated major disability survival rate of 72% for treated patients is welcome news for those diagnosed with CALD.
Zolgensma is an IV infusion indicated for spinal muscular atrophy (SMA), a rare genetic disorder characterized by degeneration of nerve cells in the brainstem and spinal cord which results in progressive muscle weakness and atrophy. SMA disorder is classified by severity and Zolgensma infusion is limited to use in patients below 2 years old and competes with Evrysdi and Spinraza. In trials, 91% of patients who had symptoms of SMA prior to Zolgensma administration achieved the milestone of being free of permanent ventilation, so they are able to breathe on their own.
Lastly, Zynteglo, also known as beti-cel, is an alternative treatment for allogeneic hematopoietic cell transplantation (HCT). It is the first gene therapy to treat an inherited blood disorder called transfusion-dependent beta-thalassemia (TDT), where the patients are incapable of producing enough hemoglobin. TDT can lead to severe anemia and the patient can experience fatigue, weakness and shortness of breath. Of the 41 patients treated in trials, 89% achieved transfusion independence.
Payment Models and Considerations
When reviewing gene therapy coverage, there are a few key challenges employers must consider.
- Therapeutic durability
As mentioned, gene therapies are one-time treatments with high upfront costs, but they can often be more cost-effective than alternative treatments over time. This can create a challenge if the long-term efficacy is insufficient. The projected savings will show in the following years as the patients stay within the plan and avoid alternative episodic treatments. - Member mobility
For employers with high turnover, the estimated savings might not materialize since they cannot calculate the cost avoidance of other competing treatments. Payors face the risk of members leaving the plan before realizing the longitudinal savings of covering gene therapy. - Robust pipeline
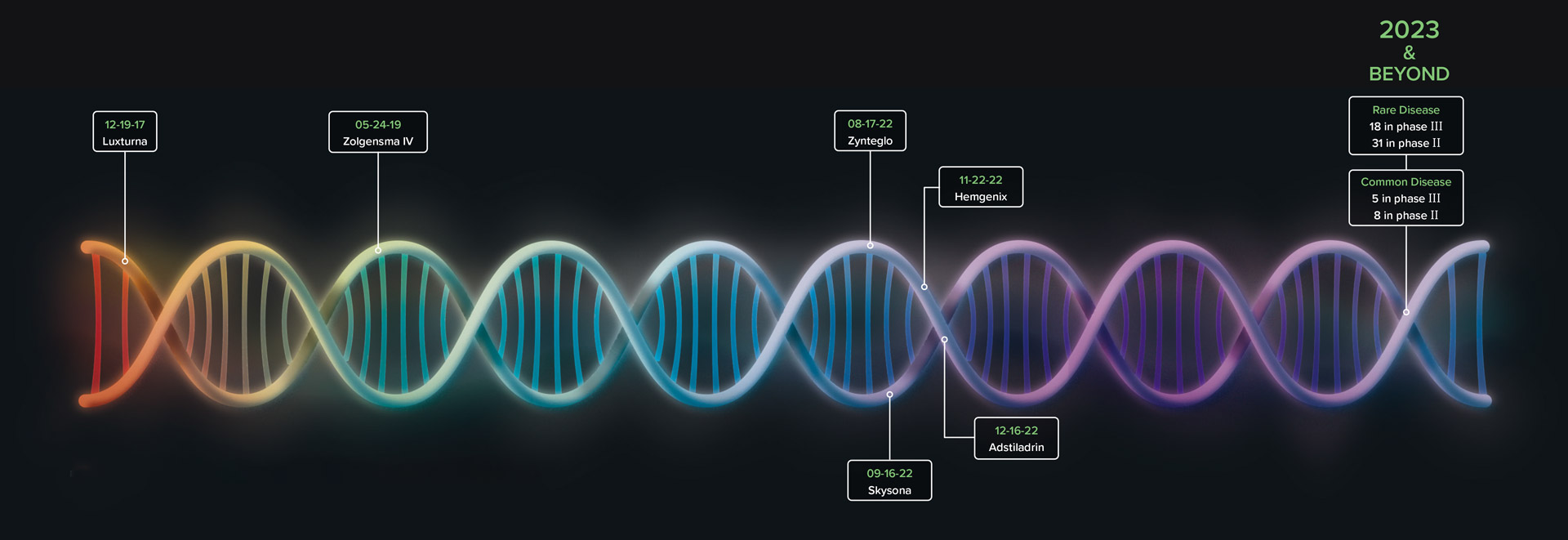
There are approximately 50-to-70 gene therapies projected to be approved by 2030. These therapies will have more than 170 indications for both rare and common diseases, which as discussed, can carry a 6-to-7 figure price tag. Clients who are considering covering the cost of these therapies must be informed and updated as they come to the market.
Due to the high price tag of these therapies, there are several reimbursement models companies are offering to provide quality access for patients.
- Outcomes-based reimbursement
Outcomes-based reimbursement models can be utilized when payors select one, upfront, lump sum payment. A specific example of this is bluebird bio implementing an innovative strategy to support access to Zynteglo. If a patient fails to achieve and maintain transfusion independence for up to two years following treatment with Zynteglo, bluebird bio will reimburse the payors up to 80% of the cost of the therapy.8 - Warranty model
The warranty model guarantees the effectiveness of the therapy to the payor for a specific time period. It outlines what health care costs should not occur when the patient is receiving the gene therapy. When the patient incurs a cost listed in the warranty, the payor receives reimbursement for the incurred cost.9 - Financial bonds
A financial bond is a risk-sharing arrangement between the payor and the manufacturer which allows the payor to make payments for therapy at the end of the treatment. However, the payor must pay interest for the treatment until the bond expires. The clinical outcome of the treatment can be part of the bond. For instance, when the patient is not responsive to the therapy, the whole bond could be lost or the payor could pay partially.
The pharmaceutical industry is constantly innovating, which also requires payors and manufacturers to innovate to determine how these exceedingly expensive therapies will be paid for. Excluding coverage of these gene therapies is a very restrictive policy that some employers may explore, but this would prevent access to these potentially life-saving therapies and leave patients to spend plan dollars on less effective care.
One way employers can protect their organizations from these expensive therapies is with stop-loss insurance. This type of insurance is utilized to manage costs associated with large claims where the risk is transferred to a reinsurer. Outside of stop loss, there are other products that protect employers from high-cost claims associated with gene therapy.
- Embarc benefit protection was launched in 2019 by Cigna and currently covers Zolgensma and Luxturna. This program is a full carve out of a per member per month (PMPM) cost requiring prior authorization approval with no member cost share.
- In 2022, UnitedHealthcare launched Optum Gene Therapy Risk Protection which also covers Zolgensma and Luxturna. This program is different in that, it requires a deductible to be met before payment of the two covered options. This program is also a full PMPM carve out and includes utilization management and prior authorization criteria.
- Lastly, CVS Caremark and Aetna have their own program referred to as the GCIT Designated Network which stands for Gene-based, Cellular and Other Innovative Therapies network. This network consists of 75 designated providers that manage how these therapies are administered and sourced. This model is a hybrid design of Aetna’s stop loss and CVS’s payment plan type.
The three products listed previously are examples of programs created by major players in the health plan and pharmacy benefits space. Quite a few start-up companies have popped up to address the risk issue for small employers, but the details on these vendors are still too new to share at this time.
The Gene Therapy Pipeline
Clinical Recommendations for Employers Health Clients
While there is a lot to unpack when it comes to this topic, approved gene therapies must be administered by healthcare professionals in highly controlled environments. Thus, the products should be exclusively covered under the medical benefit. Most of the approved gene therapies are indicated for extremely rare diseases, but the good news is, they can be very effective, especially when compared to the current treatment options. Our recommendation is to understand your risk based on your population demographics and seek vendors that have developed risk-mitigating programs, so you can have a strategy and peace of mind if a costly gene therapy claim hits your plan.
Download Insight



